PHYSICAL AND CHEMICAL WASTEWATER TREATMENT METHODS

1. Physical Wastewater Treatment Methods
- Screening is the first step at any wastewater treatment system. This process essentially involves the removal of large non-biodegradable and floating solids that frequently enter a wastewater works, such as rags, papers, plastics, tins, containers and wood. Efficient removal of these constituents will protect the downstream plant and equipment from any possible damage and pipe blockages. Wastewater screening is generally classified into either coarse screening or fine screening.
- Grit Chamber is sedimentation basin placed at the front of wastewater treatment plant to remove sand, egg shells, coffee grounds and other non putrescible materials that may clog channels or cause abrasive wear of pumps and other devices.
- In some larger plants, fat and grease are removed by passing the sewage through a small tank where skimmers collect the fat floating on the surface. Air blowers in the base of the tank may also be used to help recover the fat as a froth. Many plants, however, use primary clarifiers with mechanical surface skimmers for fat and grease removal.
- The flotation process is also widely used in industrial waste water treatment plants, where it removes fats, oil, grease and suspended solids from waste water. These units are called dissolved air flotation (DAF) units. In particular, dissolved air flotation units are used in removing oil from the wastewater effluents of oil refineries, petrochemical and chemical plants, natural gas processing plants and similar industrial facilities.
- Flow equalization is not a treatment process but a technique that improves the effectiveness of secondary and advanced wastewater treatment processes. Flow equalization levels out operation parameters such as flow, pollutant levels, and temperature over a time frame (normally 24 hr), minimizing the downstream effects of these parameters.
- Wastewater Clarifier or Sedimentation Tank plays an important role either after or before biological treatment processes to remove heavier sludge solids by means of settling and separation from the liquid phase. When it is used ahead of biological treatment, the main advantage is that it could help towards significant reduction in BOD level and thus, reducing the load feed into the aeration pond. These types of primary sedimentation tanks were designed to deal with higher rate of loading and also having shorter retention time for the water.
2. Chemical Wastewater Treatment Methods
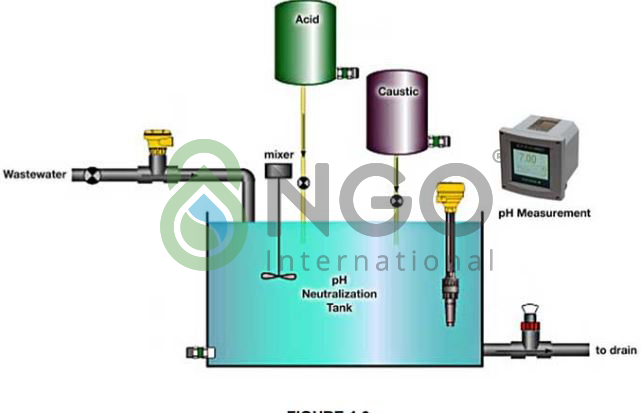
2.1 Neutralization
The purpose of neutralization is to adjust the pH value to meet the requirements of the different processing units in the wastewater treatment system.
Neutralization may be used in order to treat acid wastewaters containing metals, the method comprising increasing the pH of the acid waste by addition of an alkaline reagent, to form a precipitate and collecting the precipitate. This way the incoming solution is pH adjusted to the optimum range for precipitating metals as hydroxides.
This step is conducted before the main step of wastewater treatment, that is clarification (=decantation), to fulfill the overall wastewater treatment objectives.
2.2 Flocculants & Coagulants
Flocculation and coagulation treatment chemicals are used in effluent water treatment processes for solids removal, water clarification, lime softening, sludge thickening, and solids dewatering.
Coagulants neutralize the negative electrical charge on particles, which destabilizes the forces keeping colloids apart. Water treatment coagulants are comprised of positively charged molecules that, when added to the water and mixed, accomplish this charge neutralization. Inorganic coagulants, organic coagulants or a combination of both are typically used to treat water for suspended solids removal.
Flocculants gather the destabilized particles together and cause them to agglomerate and drop out of solution. Examples of ChemTreat flocculants include low, medium, and high molecular weight polymers.
2.3 Oxidation
Oxidation reduces the biochemical oxygen demand of wastewater, and may reduce the toxicity of some impurities. Secondary treatment converts some impurities to carbon dioxide, water, and biosolids. Chemical oxidation is widely used for disinfection.
2.4 Ion Exchange
When water is too hard, it is difficult to use to clean and often leaves a grey residue. (This is why clothing washed in hard water often retains a dingy tint.) An ion exchange process, similar to the reverse osmosis process, can be used to soften the water. Calcium and magnesium are common ions that lead to water hardness. To soften the water, positively charged sodium ions are introduced in the form of dissolved sodium chloride salt, or brine. Hard calcium and magnesium ions exchange places with sodium ions, and free sodium ions are simply released in the water. However, after softening a large amount of water, the softening solution may fill with excess calcium and magnesium ions, requiring the solution be recharged with sodium ions.
2.5 Ozonation
Ozone (O3) is applied for the disinfection of drinking water, for the removal of effluents from wastewater treatment plants in a process called ozonation (or ozonisation) as well as for the degradation of organic and inorganic pollutants in wastewater.
2.6 Disinfection
The purpose of disinfection in the treatment of wastewater is to substantially reduce the number of microorganisms in the water to be discharged back into the environment for the later use of drinking, bathing, irrigation, etc. The effectiveness of disinfection depends on the quality of the water being treated (e.g., cloudiness, pH, etc.), the type of disinfection being used, the disinfectant dosage (concentration and time), and other environmental variables.
If your business is interested in solution to inlet water treatment, or has a demand for ion exchange materials, please contact NGO via phone number (024) 3566 8225 or email office@8ngo.com for direct consultation.
thong_tin_lien_quan
-
 Wastewater treatment
Wastewater treatment
-
 A standard domestic wastewater
A standard domestic wastewater
-
 B standard domestic wastewater
B standard domestic wastewater
-
 Pig Farm Wastewater
Pig Farm Wastewater
-
 Textile dyeing wastewater
Textile dyeing wastewater
-
 Paper industry wastewater solution
Paper industry wastewater solution
-
 Textile Dyeing Wastewater & Industrial Washing
Textile Dyeing Wastewater & Industrial Washing
-
 Slaughter wastewater treatment solution
Slaughter wastewater treatment solution
-
 Aquaculture wastewater
Aquaculture wastewater
-
 Starch wastewater
Starch wastewater
-
 Petroleum wastewater
Petroleum wastewater
-
 Beer wastewater
Beer wastewater
-
 Other industrial wastewaters
Other industrial wastewaters
-
 BioPM - Organic Industrial wastewater treatment solution
BioPM - Organic Industrial wastewater treatment solution
-
 MBR solutions
MBR solutions
-
 Conventional activated sludge (CAS)
Conventional activated sludge (CAS)
-
 Moving bed biofilm reactor (MBBR)
Moving bed biofilm reactor (MBBR)
-
 Anaerobic- Anoxic-Aerobic (AAO)
Anaerobic- Anoxic-Aerobic (AAO)
-
 Sequencing batch reactor (SBR)
Sequencing batch reactor (SBR)
-
 Physical & Chemical methods
Physical & Chemical methods
-
 Supply water treatment
Supply water treatment
-
 Cooling system water
Cooling system water
-
 Water treatment for food and Beverage production
Water treatment for food and Beverage production
-
 Ultra pure water supply solution for electronic industry
Ultra pure water supply solution for electronic industry
-
 Pure water supply for pharmaceutical manufacturers
Pure water supply for pharmaceutical manufacturers
-
 Water Supply treatment with ion exchange method
Water Supply treatment with ion exchange method
-
 Ordor Treatment
Ordor Treatment


_-_NGO__2_jfif_da_sua.jpg)

