TREATING AMMONIUM IN WASTEWATER
Sources of nitrogen compound emissions into the environment are very diverse, from solid waste, emissions, wastewater, typically from the following discharge sources:
| Type of wastewater | Composition |
|
Domestic wastewater |
Source of feces and urine |
|
Industrial wastewater |
Food processing: seafood processing, slaughtering and production of seafood from foods high in protein such as meat, milk, butter, cheese, beans, mushrooms. Wastewater from slaughtering operations contains large amounts of blood, fat, feces, meat scraps, etc., Nitrogen-containing compounds are released from solid components into the water at a rate that depends on the degree of dispersion. |
|
Processing vegetables, fruits, drinks |
|
|
Flour, potato products |
|
|
Production of chemicals, fertilizers, synthetic fibers |
|
|
Agricultural and livestock wastewater |
Nitrogen and phosphate fertilizers for plants |
|
Water for cleaning barns |
|
|
Food scraps and excreta from aquaculture farms |
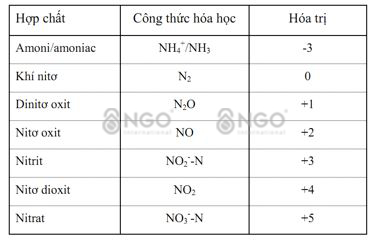
When it comes to treating Ammonium in wastewater, most people understand that it means treating NH4+ (ammonium) and NH3 (amonia) Ammonium without clearly distinguishing the difference between NH4+ and NH3. Even the current Ammonium measuring devices on the market mostly measure the TAN index - Total Amonia nitrogen, which is the sum of NH4 and NH3. NH3 is a colorless gas with a strong odor, soluble in water, and can kill fish, shrimp or aquatic animals. NH4+ is an ammonium ion, less toxic. NH3 and NH4+ always exist in parallel. In a high PH environment, NH3 dominates, in a low PH environment, NH4+ dominates. Therefore, treating ammonium in wastewater mainly involves treating ammonia NH3.
NH4(+) + H2O -->NH3 + H3O(+)
NH3 + H2O --> NH4+ + OH- (1) (ammonification)
NH4+ + 1,5 O2 --> NO2 + 2H+ + H2O (2) (nitrification)
NO2 + 0,5 O2 --> NO3 (3) (nitrification)
Organic Compound + NO3 --> Biomass + CO2 + N2↑
Ways to treat Ammonia in wastewater
- Ammonia can nourish algae and aquatic plants at certain concentrations and loadings. At aquaculture farms, to reduce Ammonia in wastewater, the farmers can actively grow algae with appropriate types and densities to absorb part of the Ammonia, reducing the impact on fish and shrimp. After that, the algae are used as food for fish and shrimp, forming a closed cycle.
- Evaporation into the air: adjust the PH in the water to high to create conditions for Ammonia in the water to exist in evaporated form, use aeration and temperature to promote Ammonia evaporation
- Oxidation:
Oxidation by microorganisms: to oxidize 1g of ammonia, 4.5g of oxygen is required. This oxidation process depends on the density of microorganisms and the oxygen concentration in the wastewater.
Chemical redox: use oxidizing compounds in appropriate proportions and under suitable PH conditions to have good ammonium treatment reaction efficiency.
Effects of ammonium on the environment and human health
- Cause eutrophication in aquatic ecosystems
- Deplete oxygen in water
- Be toxic to the microflora in water
- Increase the risk of nitrate and nitrile pollution in groundwater, affecting the community. Nitrates and nitrites can cause the cancer in humans.
With experience in consulting and implementing wastewater treatment systems using advanced and modern technology, NGO has successfully applied solutions in treating pollutants, especially Ammonium indicators. Thanks to the optimal system design, NGO has treated Ammonium concentration to meet standard A QCVN 14:2008/BTNMT for domestic wastewater.
***Vui lòng đọc kỹ yêu cầu về Điều khoản sử dụng – Bản quyền trước khi sao chép hoặc trích dẫn nội dung và hình ảnh của website.
Trang web này thuộc bản quyền của Công ty TNHH Quốc tế NGO (NGO International). Bất kỳ hình thức sử dụng hoặc sao chép một phần hoặc toàn bộ nội dung dưới mọi hình thức đều bị nghiêm cấm, trừ trường hợp được sự cho phép rõ ràng bằng văn bản từ Chúng tôi.



